In September 2020, the Food and Drug Administration (FDA) published “Requirements for Additional Traceability Records for Certain Foods” (the Traceability Rule or FSMA Rule 204), proposing regulations mandating the establishment and sharing of food production-related records to shorten the FDA’s investigations of outbreaks when they occur. After extending the original comment period until February 2021, the agency took several months to review stakeholder feedback, and the rule was finalized in November 2022. By January 20, 2026, all companies that are subject to the rule will be required to have a traceability plan in place, assign traceability lot codes and maintain records of various critical tracking events as defined in the rule. The rule comes with its own terminology that, as defined by the FDA, may or may not currently be used by or familiar to the fresh produce sector.
Planning and Preparing
Do not wait until the Traceability Rule enforcement date; prepare now. As we start 2024, two years may seem like a long time, but if companies are judiciously planning and preparing to be compliant, this time will be used to:
- Determine the information customers are going to need and the systems they use to accept that information.
- Determine the information needed from suppliers and the systems they use to log that information.
- Assess the records currently kept and the existing recording-keeping systems.
- Analyze the information shared within the supply chain and identify missing data.
During a recent Western Growers webinar, Andrew Kennedy, Principal Traceability Advisor with New Era Partners, and Minos Athanassiadis, Vice President at iFoodDS, reviewed common challenges and questions around implementing the Traceability Rule. Kennedy, who as a former FDA employee helped to write the proposed rule, outlined steps companies can take to prepare for rule compliance. An important recommendation was to follow a methodical and systematic approach to making decisions that assess the adequacy of existing recordkeeping systems as well as suppliers and customers’ information requirements and systems. Both speakers encouraged companies to do their homework and talk with their suppliers and customers first before buying new systems and/or services.
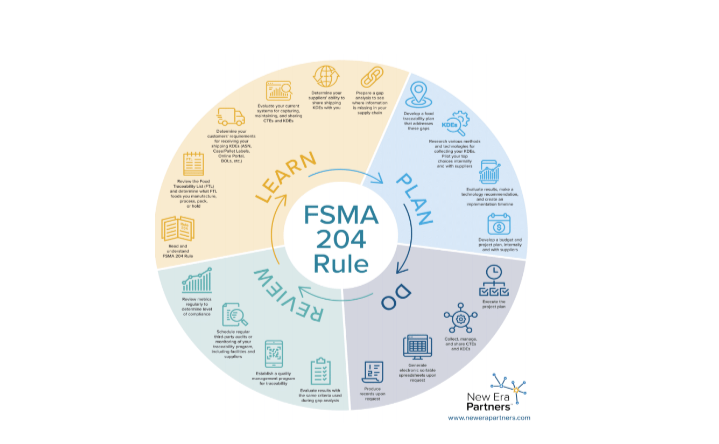
As a reference, New Era Partners/iFoods created an infographic that depicts a Food Traceability Rule Timeline and suggests a path to compliance, which includes four elements: Learn, Plan, Do and Review.
Learn means reading and understanding the Traceability Rule as well as conducting a gap analysis to see what information is missing within the supply chain. Plan means the development of a food traceability plan and developing a budget for its implementation. Do refers to having executing or implementing the traceability plan, and Review refers to assessing results, a gap analysis and periodic reviews to determine the level of compliance.
Companies that have implemented the Produce Traceability Initiative (PTI) requirements, as many on the supply side have done, are already on the path to compliance with Rule 204. Although not all Rule 204 requirements align with PTI, much of the information required by the rule is captured in the PTI-compliant case label. During the webinar, Kennedy reviewed a PTI-compliant case label and explained the elements that pertain to and align with the rule’s requirements, such as the barcode’s GTIN and internal batch or lot code, which together provide the information required in the rule’s “traceability lot code.” The PTI website includes resources, such as electronic sortable spreadsheets; a traceability implementation guide is in the works. PTI leadership is currently focused on updating its best practices related to capturing and sharing data with trading partners.
Becoming compliant with the Rule
In August 2023, Western Growers published a guidance document to help companies comply with the Traceability Rule. The guide covers: 1) which foods are and are not covered by the rule, 2) how to determine which requirements apply to you, 3) the information you are responsible for collecting and sharing, 4) how data and information will move through the supply chain, and 5) sharing information and data with the FDA. In addition, the FDA has numerous online resources available that provide an overview of the rule, highlight key features and elements, list the foods covered by the rule, review exemptions and modified requirements, and provide examples.
What is important to remember?
The Traceability Rule assigns different entities slightly different responsibilities depending on whether they are engaged in harvesting, cooling, initial packing, first land-based receiving, receiving, transforming or shipping foods on the Food Traceability List. Although “growing” is not a CTE, farms that do not engage in other activities such as harvesting, cooling or initial packing, are nonetheless required to maintain a traceability plan. FDA explicitly did not mandate that traceability records be maintained in a specific format, and covered entities are permitted to choose their preferred storage method. That said, the rule still requires traceability records to be provided in an electronic, sortable spreadsheet within 24 hours when it is necessary to address a threat to public health. For this reason, while it is not required, companies may decide it is worthwhile to store the information electronically.
For questions related to this regulation and WG resources, contact us at [email protected].